

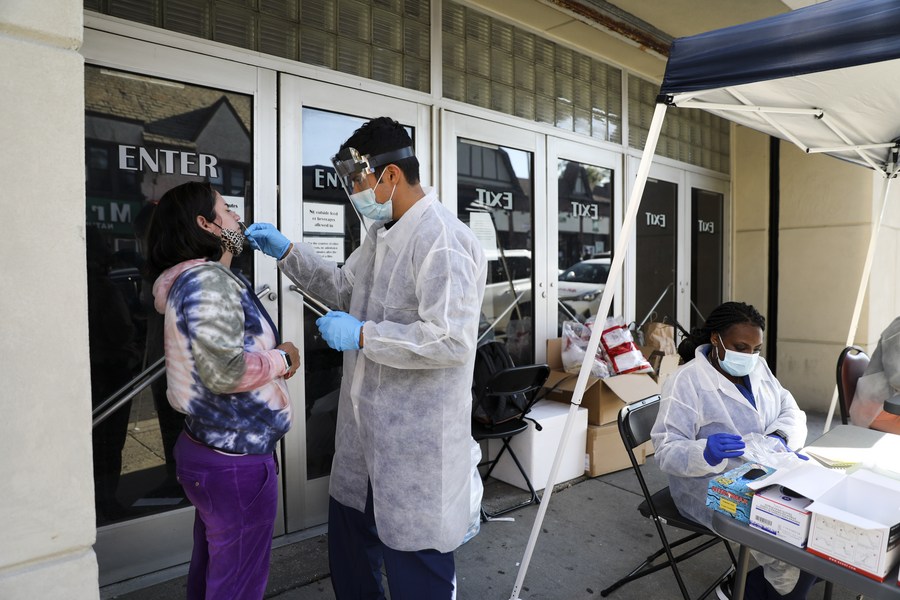
A woman gets COVID-19 test at a makeshift test site in Kew Gardens, one of the COVID-19 hotspot areas in New York City, the United States, Oct. 6, 2020. (Xinhua/Wang Ying)
WASHINGTON, Oct. 13 (Xinhua) -- U.S. drugmaker Eli Lilly said Tuesday it has paused its trial of a combination antibody treatment for COVID-19 for safety reasons.
The company said the trial's Data Safety Monitoring Board (DSMB), an independent group of medical experts who monitor clinical trials, recommended the pause.
"The trial, evaluating Lilly's investigational neutralizing antibody as a treatment for COVID-19 in hospitalized patients, is sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH)," a Lilly spokesperson said in a statement.
"Lilly is supportive of the decision by the independent DSMB to cautiously ensure the safety of the patients participating in this study," said the statement.
Neither Eli Lilly nor the NIAID, which is sponsoring the trial, have described the safety issue that prompted the decision to pause the study.

 Award-winning photos show poverty reduction achievements in NE China's Jilin province
Award-winning photos show poverty reduction achievements in NE China's Jilin province People dance to greet advent of New Year in Ameiqituo Town, Guizhou
People dance to greet advent of New Year in Ameiqituo Town, Guizhou Fire brigade in Shanghai holds group wedding
Fire brigade in Shanghai holds group wedding Tourists enjoy ice sculptures in Datan Town, north China
Tourists enjoy ice sculptures in Datan Town, north China Sunset scenery of Dayan Pagoda in Xi'an
Sunset scenery of Dayan Pagoda in Xi'an Tourists have fun at scenic spot in Nanlong Town, NW China
Tourists have fun at scenic spot in Nanlong Town, NW China Harbin attracts tourists by making best use of ice in winter
Harbin attracts tourists by making best use of ice in winter In pics: FIS Alpine Ski Women's World Cup Slalom
In pics: FIS Alpine Ski Women's World Cup Slalom Black-necked cranes rest at reservoir in Lhunzhub County, Lhasa
Black-necked cranes rest at reservoir in Lhunzhub County, Lhasa China's FAST telescope will be available to foreign scientists in April
China's FAST telescope will be available to foreign scientists in April